Using Supercritical Fluids to Remove Water from Fragile Items
Supercritical fluids are perfect for almost all Critical Point Drying applications. Carbon dioxide in its supercritical state has the permeability to reach into the smallest crevices of any artifact. Unlike liquids, Supercritical CO2 has no surface tension to destroy the artifact you’re trying to restore.
With ‘normal’ evaporation techniques as the water goes from liquid to gas, the surface tension created by this state change pulls against the structure it is attached to causing capillary stress, and in the case of delicate artifacts, often destroying the artifact.
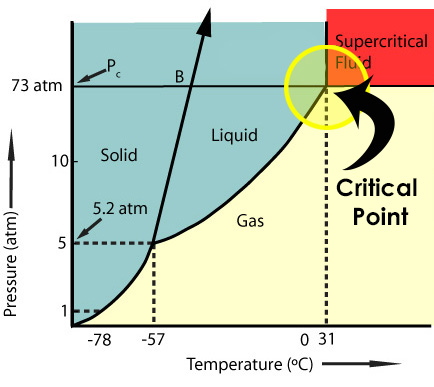
Using supercritical fluid to remove the liquid from an organic artifact allows you to do so with no surface tension. Critical point drying with supercritical CO2 involves heating/pressurizing the CO2 past its critical point.
At its critical point, supercritical fluid loses all surface tension. The supercritical fluid can now be removed from the artifact by depressurizing while maintaining a temperature above the critical temperature. As pressure is released, molecules are removed as gas and the fluid becomes less dense. After enough of the fluid has been removed, the vessel can be allowed to cool and the remaining liquid will convert to gas, thereby removing the water from your artifact with no damage.